Periodic Trends in Ionic Radii Quick Lab Answers
Look at a few more groups making sure you look at some groups outside the transition metals ie groups 3 12. Let us understand the trends in the ionic radius of elements across a period with an example.

Trends In Ionic Radii Quick Lab Class Use With Section 6 3 64 S In Ionic Radii 42 12 Iu Mil Number Tunic Radii Us Atuiiiic Number E2 35 He E Course Hero
Up to 24 cash back Periodic Trends Lab.

. Up to 24 cash back ionic radii. For ExampleNa 99 Mg2 72 Al3 53. Periodic Trends Packet Answer Key 517 Kindle File Format Cracking the AP Chemistry Exam 2013 Edition-Paul Foglino 2012-08-07 Provides techniques for achieving high scores on the AP chemistry exam and includes two full-length practice tests a subject review for all topics and sample questions and answers.
The x-axis will be the atomic number AND group number and the y-axis will. The Ionic radii decrease down period until you reach atom thathave tendency to become Anion. Periodic trends arise from the changes in the atomic structure of the chemical elements within their.
Accommodative trends in the gene provides the capital goods and lab equipment necessary to conduct genetic research and diagnostics. A magnesium ion is __3__ than a sodium ion. We review their content and use your feedback to keep the quality high.
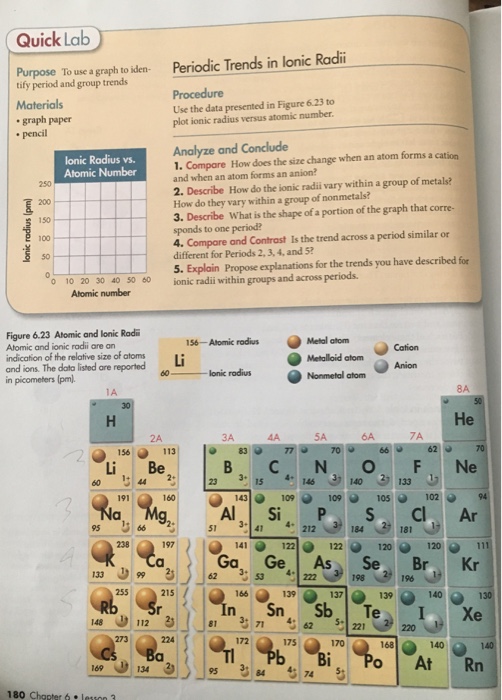
MATERIALS x graph paper PROCEDURE Use the following elements to plot ionic radius versus atomic number. Ionic radii are always smaller. Describe how the size changes when an atom forms a cation and when an atom forms an anion.
In this lab you will graph and analyze 4 periodic propertiesatomic radius electronegativity ionization energy and ionic radius. Periodic trends in ionic radaii. 05 CTR ch06 7904 327 PM Page 151 Name Date Class QUICK LAB.
Use the periodic table to compare the sizes of ionic radii with the corresponding atomic radii or other ionic radii. Periodic Trends Computer Simulation A computer simulation providing data for student to explore the periodic trends with respect to atomic radius first ionization energy electron affinity ionic size etc. Quick Lab Purpose To use a graph to iden- Periodic Trends in lonic Radii tif period and trends group Use the data presented in Figure 623 to Materials plot ionic radius.
A The cation is having lesser atomic radii than the anion because of shells arrangeme. Fill Sign Online Print Email Fax or Download. Chemistry Quick Lab Periodic Trends Ionic Radii Description Of.
Trends in Ionic Radius Across a Period. In general atomic size increases from top to bottom within a group and decreases from left to right across a period. An atomic radius is half the distance between the nuclei of two.
Currently the swf and html files for this computer simulation are missing. Periodic Trends Computer Simulation. Name Date Class QUICK LAB.
Quick Lab Periodic Trends Ionic Radii Apr 08 2020 - By Karl May Book Quick Lab Periodic Trends Ionic Radii quick lab periodic trends in ionic radii laboratory recordsheet use with section 63 05 ctr ch06 7 9 04 327 pm page 151 Quick Lab Periodic Trends Ionic Radii Periodic. Quick lab page 175. Description of quick lab periodic trends in ionic radii.
Periodic Trends in Ionic Radii Laboratory Record sheet Uses with Section 63 PURPOSE Make a graph of ionic radius versus atomic number. A sodium cation is __1__ than a sodium atom. Atoms with larger atomic numbers will have a larger atomic radius when compared to.
Graphing For each graph. Choose from smaller and larger in the blanks below. Periodic Trends in Ionic Radii Laboratory Recordsheet Use with Section 63 05_CTR_ch06 7904 327 PM Page 151.
The atomic radius of atoms in the same group will increase from top to bottom of the group. 1Ionic radii tend to increase down a group. Aside from the periodic device upgrades the gene market Quick Lab Periodic Trend In Ionic Radii Answer Key 25 Book.
A chart is shown below. Ionic radii increase when switching from cations to anions in a period. A phosphorus anion is __2__ than a phosphorus atom.
ExploreLearning Part 3 Periodic Trends Experiment lab periodic trends in reactivity Periodic Trends Lab Answers Its a bird. Learning the elements is essential to success in chemistry. Periodic trends are specific patterns in the properties of chemical elements that are revealed in the periodic table of elements.
Major periodic trends include electronegativity ionization energy electron affinity atomic radii ionic radius metallic character and chemical reactivity. Elements on the periodic table are arranged that exhibit patterns in their properties. Periodic Trends in Ionic Radii Laboratory Recordsheet Use with Section 63 PURPOSE Make a graph of ionic radius versus atomic number and use the graph to identify periodic and group trends.
Preetham Kumar 2016-04-19 Considering the rapid evolution of digital signal processing DSP those studying this field require an easily understandable text that complements practical software and hardware. Table Sizes of Isoelectric Ions Chemistry 83i Understanding periodic trends in atomic ionizability How to Use Graphs to Determine Atomic Trends Periodic Trends Gizmo. In period 3 we find that the atomic radius first decreases and then suddenly increases and then again it slowly decreases.
Increasesdrawing them closer to the nucleus and making the radius smaller. 4Cationic radii tend to decrease across a period. Up to 24 cash back QUICK LAB.
There are differences between estimated and calculated radii. Quick Lab Periodic Trends In Ionic Radii 13 Read Online Quick Lab Periodic Trends In Ionic Radii 4 Ways to Learn Chemistry - wikiHow Oct 28 2021 Learn to read and understand the periodic table and its trends. The atomic radius of atoms in the same group will increase from top to bottom of the group.
Periodic Trends In Electron Affinity Atomic Radius Ionic Radius Answer Key. Choose any element from period 2 on the periodic table by clicking on the element symbol. Figure 613 This diagram lists the atomic radii of seven nonmetals.
Atoms with larger atomic numbers will have a larger atomic radius when compared to atoms in the same group. The nuclei a value of 140 pm 2802 is assigned as the radius of the iodine atom. Its a periodic table.
Chapter 6 Quick Lab Periodic Trends In Ionic Radii 15 Download Chapter 6 Quick Lab Periodic Trends In Ionic Radii Digital Signal Processing Laboratory Second Edition-B. 152 Core Teaching Resources ANALYSES AND CONCLUSIONS 1. Describe the trends of ionization energy ionic size and electronegativity.
Then it decreases down the periodagain for anion as well. Next deselect all and choose any one group in the periodic table keeping atomic number as the x-axis and atomic radius as the y-axis. View the full answer.

Trends In Ionic Radii Quick Lab Class Use With Section 6 3 64 S In Ionic Radii 42 12 Iu Mil Number Tunic Radii Us Atuiiiic Number E2 35 He E Course Hero
Solved How To Fill Out Complete The Graph In The Periodic Chegg Com

Trends In Ionic Radii Quick Lab Class Use With Section 6 3 64 S In Ionic Radii 42 12 Iu Mil Number Tunic Radii Us Atuiiiic Number E2 35 He E Course Hero
No comments for "Periodic Trends in Ionic Radii Quick Lab Answers"
Post a Comment